
Lyophilization: cycle robustness and process tolerances, transfer
Price: $ 2963.00
4.8(295)
During the past 10-15 years, close attention has been paid to the development of optimal lyophilization cycles for different types of pharmaceuticals1-4. Recent advances in process control, such as the Smart Freeze-DryerTM technology or similar approaches, [5-7] make cycle development a routine procedure. The attention of many researchers has shifted to the aspects of cycle transfer and scale up that still require significant investment in understanding the differences in lyophilization processes between laboratory and commercial dryers [8-14].

A model-based optimization strategy to achieve fast and robust freeze-drying cycles - ScienceDirect

Design Space Development for Lyophilization Using DOE and Process Modeling
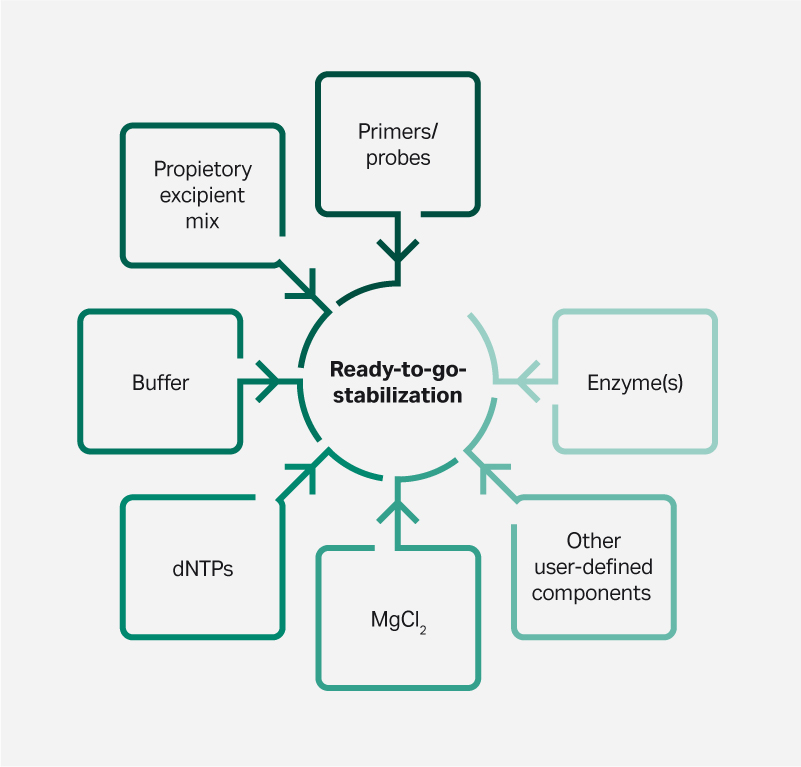
Advantages of lyophilization

Recommended Best Practices for Lyophilization Validation—2021 Part I: Process Design and Modeling

Practical Considerations for Freeze-Drying Process Design, Development and Scale-Up American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
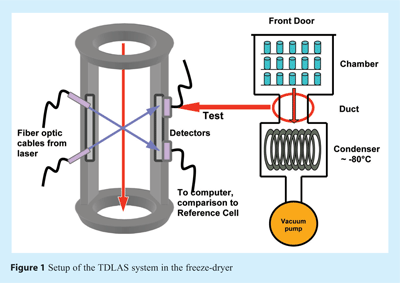
Process Analytical Technology (PAT) in Freeze Drying: Tunable Diode Laser Absorption Spectroscopy as an evolving tool for Cycle Monitoring - European Pharmaceutical Review

A model-based optimization strategy to achieve fast and robust freeze-drying cycles - ScienceDirect

Accelerating the Development and Transfer of Freeze-Drying Operations for the Manufacturing of Biopharmaceuticals by Model-Based Design of Experiments

Accelerating the Development and Transfer of Freeze-Drying Operations for the Manufacturing of Biopharmaceuticals by Model-Based Design of Experiments

Lyophilization Process Understanding and Scaleup Using Ab Initio Vial Heat Transfer Modeling

The freezing step in lyophilization: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals - ScienceDirect

Principles and Practice of Lyophilization Process and Product Development: Scale-Up and Technology Transfer

Lyophilization Validation: Process Design and Modeling




